GenoPredicta™
A cutting-edge genomic test for myeloma from blood or marrow
| FISH | Marrow | Blood | |
|---|---|---|---|
| Fresh or frozen samples | ✓ | ✓ | ✓ |
| Minimally invasive | ✗ | ✗ | ✓ |
| Cell requirements | 50/probe | ~50 | ~50 |
| All genetic alterations* from WGS | ✗ | ✗ | ✓ |
| Satisfies new IMS high-risk criteria (TP53 sequencing) | ✗ | ✓ | ✓ |
| Detects mutations/deletions of therapeutic targets | ✗ | ✓ | ✓ |
| Discovers new resistance markers | ✗ | ✓ | ✓ |
| Representative of all lesions/clones, not just sampled site | ✗ | ✗ | ✓ |
| Monitoring of treatment response (clonal evolution) | ✗ | ✗ | ✓ |
| Other blood cancers | ✗ | ✗ | ✓ |
| All disease states | ✗ | ✗ | ✗ |
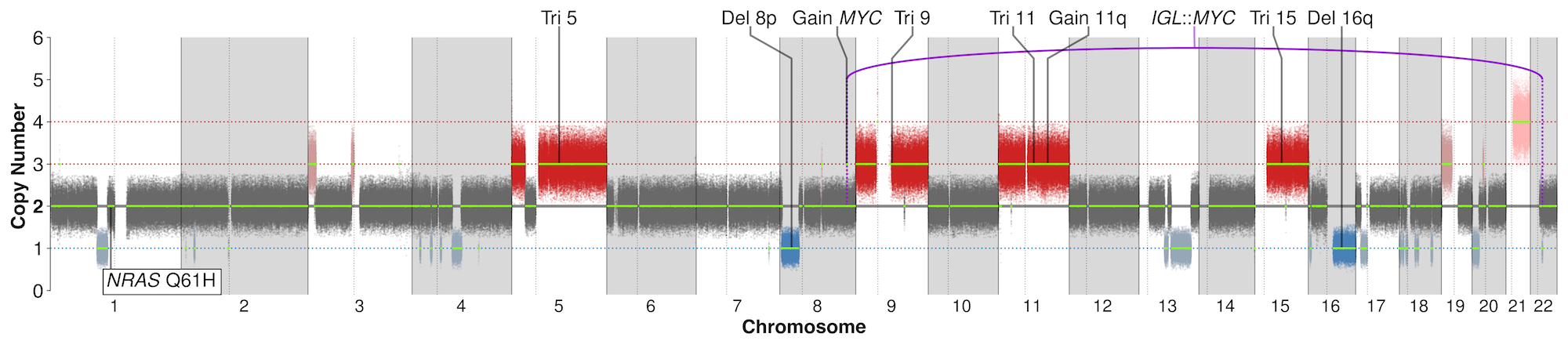
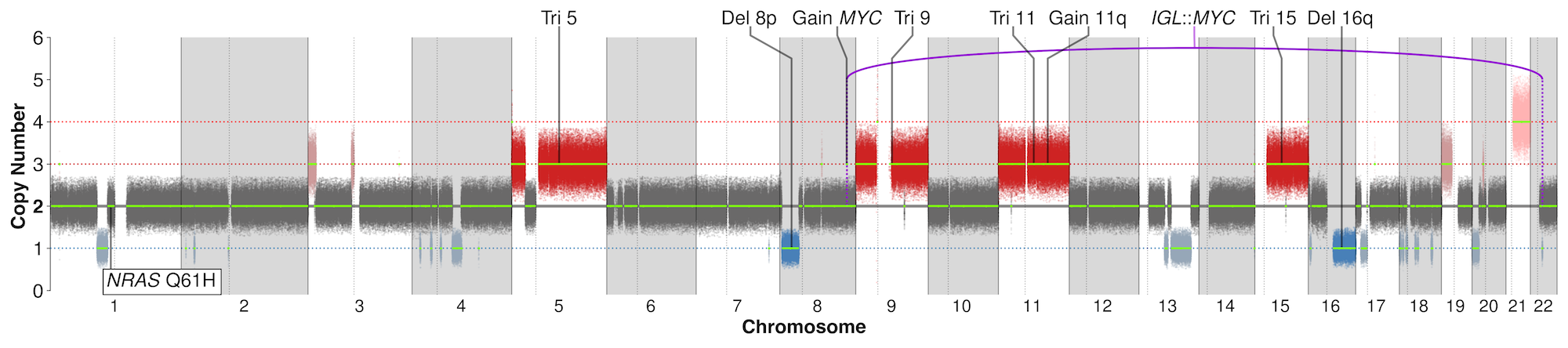
- GenoPredicta™ for bone marrow or blood is a WGS-based assay and therefore can identify SNVs and short insertion/deletion (InDel) variants in therapeutic targets, including TNFRSF17 (BCMA) and GPRC5D.
- However, GenoPredicta™ currently does not detect other resistance mechanisms, including downregulation of TNFRSF17/GPRC5D or epigenetic silencing of GPRC5D.
Yes, it satisfies the requirements set by the International Myeloma Society/International Myeloma Working Group (IMS/IMWG) guidelines that mandate the use of next generation sequencing to identify patients with high-risk MM (Avet-Loiseau et al., Journal of Clinical Oncolology, 2025).
The reported somatic variants include prognostic cytogenetic variants that are currently tested in common practice using FISH assays, as well as other clinically relevant genetic alterations in MM, including:
- Structural variants (SV) – Genomic translocation events including IGH translocations such as IGH::CCND1 t(11;14), IGH::FGFR3/NSD2 t(4;14), IGH::MAF t(14;16), and IGH::MAFB t(14;20).
- Copy number variants (CNV) – Large chromosomal gains/losses such as Del1p (CDKN2C loss/deletion), 1q+ (CKS1B gain/amplification) and Del17p (TP53 loss/deletion), as well as small focal events, such as TP53 deletions.
- It also identifies mutations/alterations in TP53 and biallelic deletion of 1p32, which are part of the IMS/IMWG guideline requirements but cannot be detected by FISH.
- Del(17p) and/or TP53 mutation*
- t(4;14) or t(14;16) or t(14;20) and 1q+ and/or del(1p32)
- Monoallelic del(1p32) and 1q+, or biallelic del(1p32)*
*WGS required

-
What type of sample is required?We can use either a blood sample or bone marrow. For fresh peripheral blood, the volume is ideally up to 30 ml, with a minimum of 10 ml volume required. The minimum requirement for fresh bone marrow aspirate is 2 ml in one EDTA tube.
-
How do I order a test?
- Complete the GenoPredicta™ Test Requisition Form (TRF).
- Email genopredicta@predictabiosciences.com to request a copy of the TRF.
- A completed TRF must accompany all samples.
- Licensed Medical Professionals may also submit TRFs online via Docusign.
- Samples must be sent to Predicta's laboratory at
Predicta Biosciences
within 24 hours of specimen collection.
750 Main Street, Lab 413
Cambridge, MA 02139
-
What supporting documentation is needed?We request clinical notes (including treatment history, prior histologic, and cytogenetic findings, as well as a FISH report, if available) to aid in the pathologist’s review.
-
Which patients are ideal candidates for GenoPredicta™?GenoPredicta™ is suitable for:
- Newly diagnosed patients
- Relapsed/refractory patients
- Patients being considered for clinical trials
- For diagnosis, monitoring and therapy selection purposes
-
How long does it take to receive the results?The average report is delivered within 14-28 days.
-
What is the cost of the test?GenoPredicta™ is in its Early Access Program, so there are currently no fees.
-
Are you CLIA certified?Predicta Biosciences is currently CLIA-approved. Our CLIA number is 22D2314039.
-
Where can I find more information?For additional inquiries, contact genopredicta@predictabiosciences.com
The right test
for the
right treatment
GenoPredicta™: a more accurate and sensitive way to test myeloma genetics in both the bone marrow and blood

The GenoPredicta™ test can use either bone marrow or a blood sample, and is a more accurate, sensitive way of profiling your myeloma — helping doctors better understand your disease and how it might respond to different treatments. GenoPredicta™ uses a powerful method called whole genome sequencing to look closely at the DNA of cancer cells. When your doctor orders a bone marrow biopsy, this can provide more accurate and sensitive information than FISH.
With an advanced test like GenoPredicta™, your care team may be able to make more personalized and effective treatment decisions — right from the start. For example, it can find changes in your genes that might stop certain treatments, like CAR-T therapy, from working properly.
GenoPredicta™ is designed to improve how we understand and manage multiple myeloma — helping doctors make more accurate predictions and choose better treatments for you.

- Ask your doctor to order the GenoPredicta™ test.
- Your provider will order the GenoPredicta™ test from either a bone marrow or blood sample.
- Predicta Biosciences will receive your sample, run the test, and send the test results back to your doctor as a clinical report.
- Your doctor will share the genetic information with you and how it changes your therapy or your prognosis.
-
What kind of sample do I need to provide?We offer the option of using either a blood or bone marrow sample. GenoPredicta™ is an advanced test that provides results from blood with similarly high accuracy as from bone marrow, but with less discomfort and invasiveness.
-
What will the GenoPredicta™ report tell me?The report will show genetic changes that have been detected in your myeloma cells. It may also include information about how your cancer might respond to different treatments.
-
Is this test available in my state?The test is available in all states except New York. We’re actively working to expand access.
-
How does my doctor order the test?The test must be ordered by a licensed medical provider, who will need to complete a Test Requisition Form (TRF). You can get the form here or by emailing genopredicta@predictabiosciences.com. A test kit is required to complete the TRF and will be mailed to you and/or your provider. Licensed medical professionals can also submit TRFs online using Docusign. Sending a copy of the TRF in advance is optional, but it can help our lab team with planning.
-
Do you need my medical records?We request recent clinical notes/summary (including treatment history, prior histologic, and cytogenetic findings, and FISH results if available) to take into consideration in the GenoPredicta™ report and aid in the pathologist’s review.
-
Do I need to include my insurance information?Predicta has launched an Early Access Program for its GenoPredicta™ assay. For the duration of this program, Predicta will bill neither public nor private insurers.
-
How much does the test cost?Currently, GenoPredicta™ is part of an Early Access Program, so there is no cost for patients.
-
How long will it take to get my results?The average report will be delivered within 14-28 days.
-
How can I learn more?Contact us at genopredicta@predictabiosciences.com for any questions.

